Medical Device Fabrication Schemes
In the context of translational research into products for health and medicine, our department “Medical Device Fabrication Schemes” deals with manufacturing processes. For promising medical product candidates, we develop manufacturing schemes in which the various steps of future production are played out. To this end, we research and create integrated synthesis and shaping processes , clean room technologies and sterilization techniques. This research work also includes structural and purity analysis. Medical device candidates can then be evaluated in (pre-)clinical studies using qualified test devices on this basis.
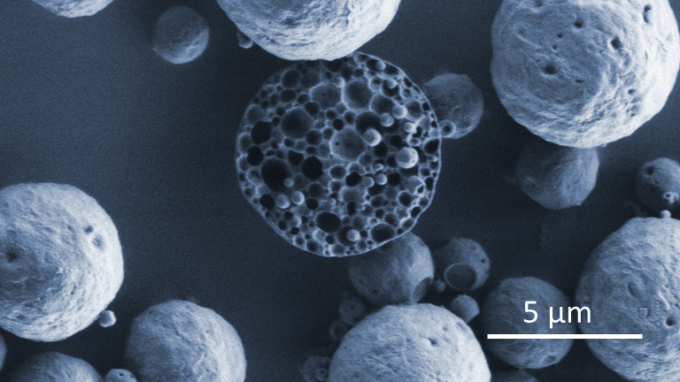
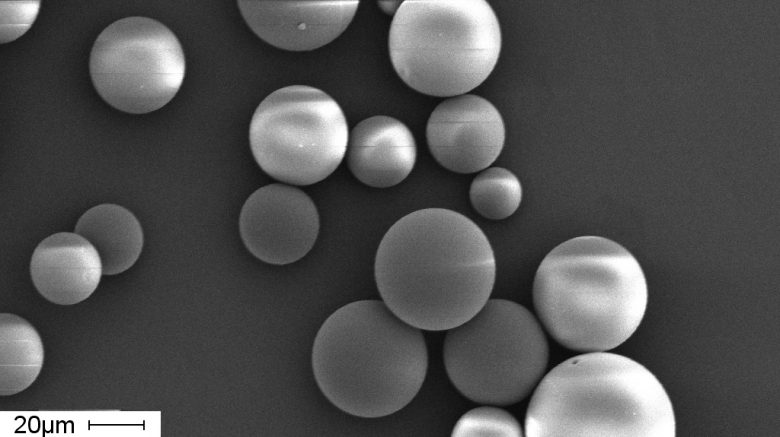
Microparticle carriers with porous inner structure encapsulating bioactives (SEM image) Photo: Hereon
Pharmaceutical Technology is the science that transfers and incorporates bioactive substances into so-called dosage forms or formulations which are suitable for administration. In this way, bioactive molecules can be properly delivered and become an effective treatment of human diseases.
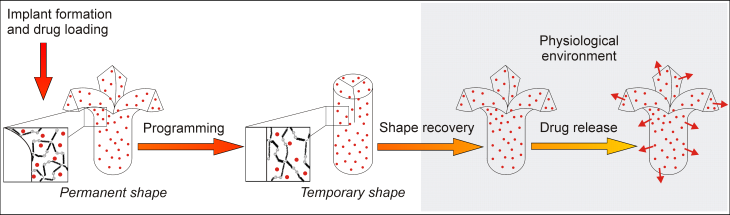
The research group Pharmaceutical Technology focuses on delivery systems based on polymers. We particularly address systems used for parenteral administration such as implants or injectable particles. New polymeric materials and new concepts for building carrier systems are the main strategic approaches followed to establish a controlled delivery.
Modern carrier systems should combine functions relevant for the respective biomedical application which leads to a demand of multifunctionality. Most importantly, the different functions of the delivery systems should be controlled independently from each other. For example, carriers may exhibit stimuli-sensitivity, thus being capable to respond to externally applied triggers. Such drug carriers may carry bioactive molecules ranging from conventional drugs to proteins and release them continuously or on-demand.
Profile
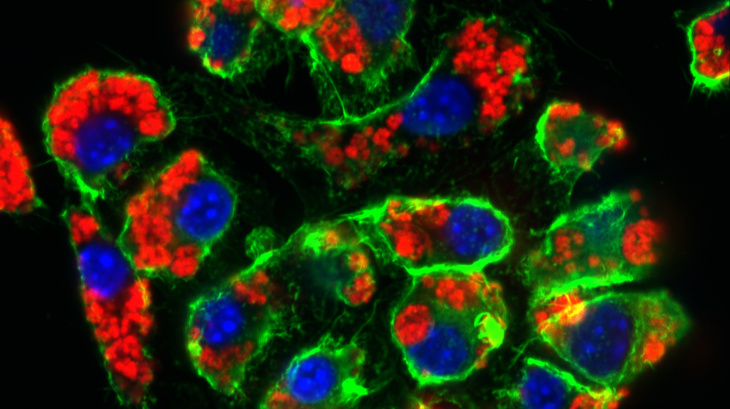
Nanoparticles (red) taken up by macrophages within 24 hours (RAW-Blue Cells, cell nucleus blue, cell skeleton green) Photo: Hereon
The research group Pharmaceutical Technology develops controlled delivery systems for bioactive molecules to be used in parenteral administration. These systems are based on polymers ranging e.g. from hydrophobic (co)polyesters to very hydrophilic materials. From such polymers, a variety of drug carriers can be prepared.
In addition to research based on new materials and new carrier concepts for controlled delivery, the research group also addresses further key elements of translational biomaterial research such as sterilization of carrier systems and aseptic preparation in a GMP conform clean room.
Variety of Carrier Systems
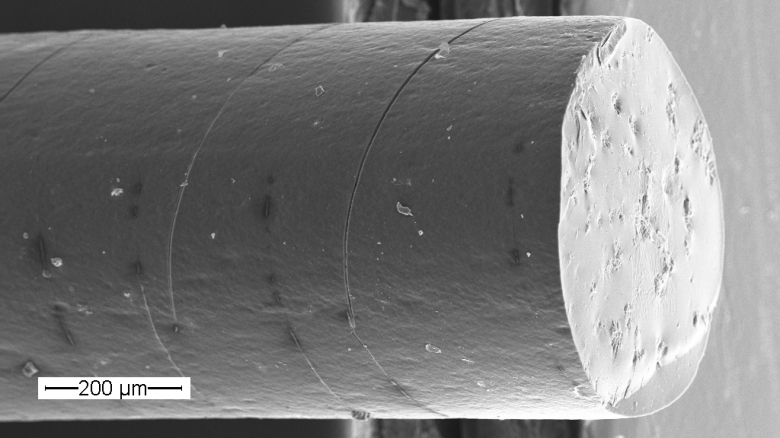
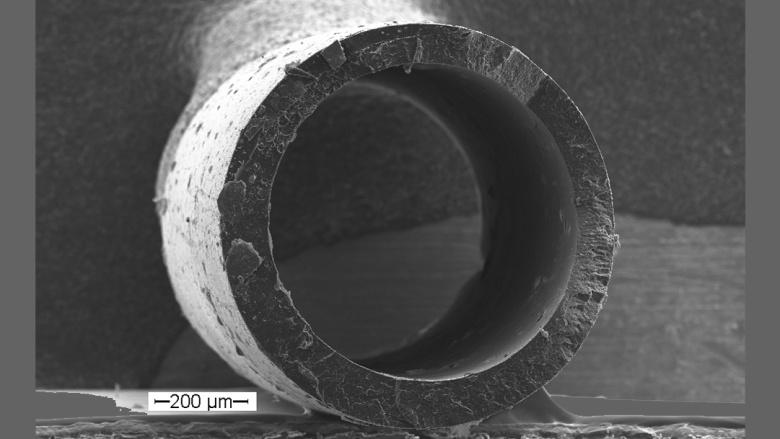
The selection of a suitable carrier systems requires consideration of demands set by the specific application and the properties of the bioactive substances. The studied carrier systems range from injectable micro/ nanoparticles to preformed implants like rods or polymeric stents, in situ forming implants, or hydrogels.
New Polymer Systems and Technologies for Pharmaceutical Sciences
The work of the research group Pharmaceutical Technology includes new concepts for drug carriers which are driven by the material development in this group and other departments of the Institute of Biomaterial Science. Tailorable properties and functions of new polymers are a cornerstone for establishing novel systems for controlled delivery.