Sensors for pH and Alkalinity
Shelf seas are a critical interface between the land and the open ocean. Consequently, processes in shelf seas play a crucial role in global biogeochemical and carbon cycles and have a significant influence on ocean CO2 storage. Continuous monitoring of the carbon cycle in the ocean is highly relevant to understanding climate change and ocean acidification. To fully quantify the complete carbon system in seawater it is necessary to determine at least two of the five variables of the carbon system in seawater. The Helmholtz-Zentrum Hereon has therefore developed autonomous underway systems with a high temporal resolution (1/min) for alkalinity and pH value.
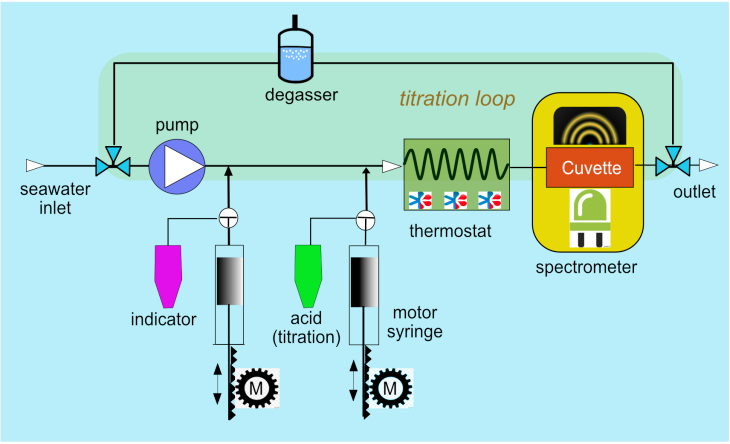
Schematic overview of the measurement principle for alkalinity. -image: Friedhelm Schroeder / Hereon-
The pH analysis system adds an acid-base indicator dye to a seawater sample. The indicator dye’s extinction coefficients change with the acidity of the water sample. This principle can be used for high precision spectrophotometric determination of the pH value. One big advantage of this method is that no calibration of the indicator is needed in the field as no drift occurs. Only temperature and salinity have to be known accurately.
To determine total alkalinity, a tracer-monitored titration with a strong acid is performed. The tracer (bromocresol green) allows to optically measure the concentration of the indicator and, hence, the concentration of the acid. In addition, the pH value can be calculated during the titration procedure, which is needed to determine the alkalinity.
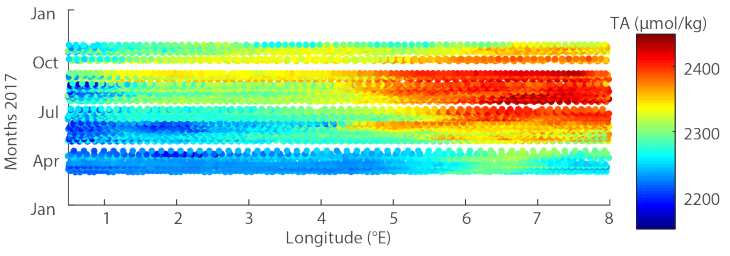
Weekly FerryBox measurements of alkalinity on the route Cuxhaven- Immingham in 2017. -image: out of ferrydata.hzg.de / Hereon-
| pH | total alkalinity |
