Hydrogen Storage: new tank for EcoBee
On Friday, July 3rd, Institute Director Prof. Thomas Klassen delivers the tank developed by scientists at the HZG. A student team, "Fortis Saxonia", at the University of Chemnitz undertakes the integration of the tank into the “Umicore EcoBee”, a vehicle built in 2014.

Umicore EcoBee concept car runs on new hydrogen storage tank developed at the Helmholtz-Zentrum. Photo: HZG/ Heidrun Hillen
“We’ve been using different vehicles developed by the Chemnitz students to test our metal hydride storage under real conditions since 2006,” explains Prof. Thomas Klassen, Director of the Materials Research Institute, where Klassen oversees the Materials Technology Division. “Through this cooperation, we have already gained valuable knowledge for further developing our technology.” This cooperation continues with the students' sixth car.

Institutsleiter Thomas Klassen
Photo: HZG/ Julia Knop
EcoBee is a vehicular concept informed by nature. Thus, for example, the “EcoBee” is designed to look and operate sustainably. The vehicle runs on hydrogen and produces electricity in the fuel cell for the electric motor. The exhaust gas developed is water. The vehicle, only 180 kilograms in weight with a CFRP chassis, can reach a maximum speed of approximately fifty kilometres per hour. The tank can store approximately one thousand litres of gaseous hydrogen. This is equivalent to the energy content of about 0.34 litres of gasoline.
The metal hydride Hydralloy C5 comprises the foundation of the tank. This metal powder, based on manganese and titanium, was specially treated and processed by researchers from the Nanotechnology Department in Geesthacht so that it can be used for storage.
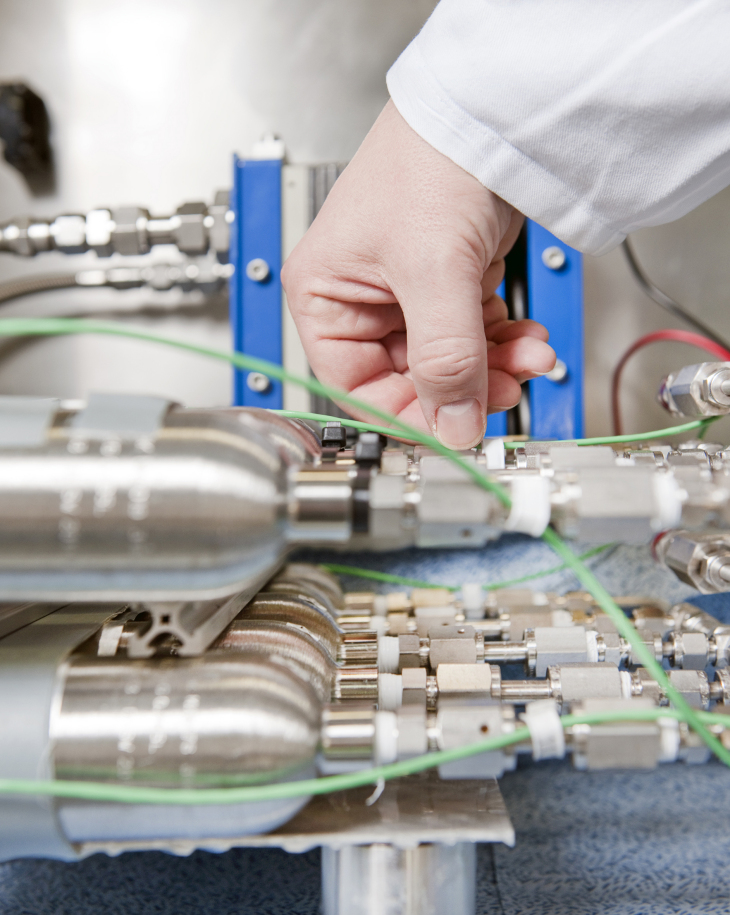
Testen des neuen Tanksystems im Wasserstoff-Labor der Geesthachter Forscher.
Photo: HZG/ Julia Knop
For this purpose, the scientists in Geesthacht possess their own laboratory space in which the tank was tested beforehand. The powder is ground to the desired nanostructure in a high-energy ball mill. This increases the surface area of the base material used for storage, which can thus take up more hydrogen faster.
An additional development is that, in conjunction with the vehicle’s fuel cell system, the waste heat can be used for the storage tank by employing constructive measures. With this additional waste heat, the fuel cell’s necessary input pressure can be sustained longer, even during peak load. The new storage thus uses the fuel cell's waste heat for more efficient unloading (desorption) because the tank cools naturally during this process.
“We are excited to see what ground the EcoBee will cover with its new tank,” says Martin Rößler, executive committee member of Fortis Saxonia. "The preceding laboratory experiments have shown that the tank's capacity has considerably increased.”
Further Information:
Fortis Saxonia: Fortis Saxonia is a student research project at the Technical University Chemnitz for developing lighter and more energy-efficient vehicles. Fortis Saxonia is a registered non-profit organisation and is financed through sponsorship and donations.
Institute of Materials Research, Division of Materials Technology at the Helmholtz-Zentrum Geesthacht: This institute division is directed by Prof. Thomas Klassen, who is also professor at the Helmut Schmidt University Hamburg. The institute’s staff, approximately thirty in number, study selected hydrogen and nanotechnology applications. Their work is interdisciplinary and trans-departmental and includes the study of nanostructured semi-conductor surfaces for the photoelectrochemical production of hydrogen by means of sunlight. Their work also encompasses the development, optimization, production and evaluation of new storage materials, hydrogen tank design and testing as well as investigation of nanostructured thin layers for X-rays and neutron optics.
Metal Hydride Storage: A fine nanocrystalline powder made of a suitable metal alloy forms the tank’s storage medium. The gaseous hydrogen is fed into the tank under pressure (max. 100 bar) and forms a metal hydride with the storage medium. This reaction is reversible: the heat is released (exothermic reaction) during loading (absorption), and the heat must be supplied by the environment (endothermic reaction) during unloading (desorption).
Kontakt

